HOME >> CHINA
Doubts rise over virus vaccine
By GT staff reporters Source:Global Times Published: 2020/2/25 20:43:40
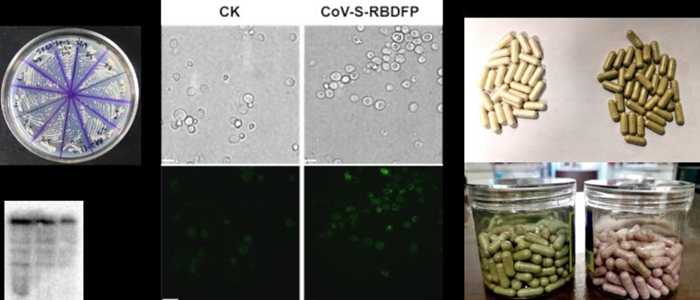
COVID19 oral vaccine. Photo: Courtesy of Tianjin University
While dozens of medical companies worldwide are stepping up research and development of a vaccine for the novel coronavirus, a university in North China announced Tuesday that they had successfully developed one, triggering doubts over the product's effectiveness, given it came just less than two months after the outbreak.
Tianjin University said on Tuesday that a research team under the university had developed an oral vaccine utilizing food-grade saccharomyces cerevisiae as a carrier and the spike protein of the coronavirus as a target spot to produce antibodies to fight COVID-19.
Huang Jinhai, the professor who led the project, said he has taken four doses and has not yet experienced any side effects, according to media reports.
The news soon made waves on Chinese social media, with some respecting the scientists' efforts while others questioning the effectiveness as the drug was developed within a very short period of time and the professor did not provide sufficient evidence to prove its reliability.
The National Medical Products Administration told media on Tuesday that all vaccines must undergo clinical tests, in response to the Tianjin University's development.
"It is possible to develop a vaccine in one month, but impossible to complete animal experiments in such a short time, which are vital to prove the effectiveness of the vaccine," Yang Zhanqiu, deputy director of the pathogen biology department at Wuhan University, told the Global Times on Tuesday.
In a special period with green channels provided by related authorities, it takes at least six months, Yang said.
Without clinical trials, the effect cannot be verified, and it is not known whether the virus will suggest it. Like the flu virus, it varies from year to year, so the vaccine from the previous year is basically useless, a frontline doctor surnamed Cheng who works at Wuhan Tongji Hospital told the Global Times on Tuesday.
Yang also said the oral drug Huang's team developed may be different from preventative vaccine people usually take.
"Preventive vaccine is usually injected. Huang's product may be a kind of therapeutic vaccine, which is just like a drug. But what we urgently need is preventative vaccine to protect healthy people from the virus," Yang noted.
Huang noted that he has developed a similar animal vaccine for COVID-19 and that the vaccine has proven to be effective, which led him to deduce the vaccine could be effective on humans.
He explained that the vaccine could be developed within a short period of time because his team has been building a biological prevention and formulation development platform based on food-grade saccharomyces cerevisiae for years.
For the next step, Huang said he is looking for qualified enterprises to work together with his team to follow evaluation procedures, so as to speed up the clinical verification process and expand the vaccine's use.
An insider in the medical industry told the Global Times that, as far as he knows, more than 40 vaccine development projects have been launched in China since the outbreak, but most of them are probably just hype for company performance or stock market reporting.
There is still a long way to go for clinical use, although the basic claims and mechanisms are not wrong, Cheng said, echoing the insider.
But viruses are also cunning, often changing their structure to evade the immune system and prevent you from identifying them, Cheng said, citing the influenza virus and the HIV virus.
Each year, several tens of billions of dollars are used on research of these viruses, but no vaccine has come out. So it's easy to just say some theories, and it is doubtful whether they are effective, she said.
Li Lanjuan, a member of the Chinese Academy of Engineering, said in an interview with media last month that her lab was accelerating vaccine development but still needs at least three months from isolating a virus to approve a vaccine.
Meanwhile, Boston-based biotech start-up Moderna announced Monday that they had sent a vaccine on the novel coronavirus they developed to the US National Institutes of Health to be tested on humans. A clinical trial on the vaccine of about 20 to 25 healthy volunteers is expected to start in April, with initial results available in July and August, media reported.
Newspaper headline: Doubts rise over COVID-19 virus vaccine
RELATED ARTICLES:
Posted in: SOCIETY