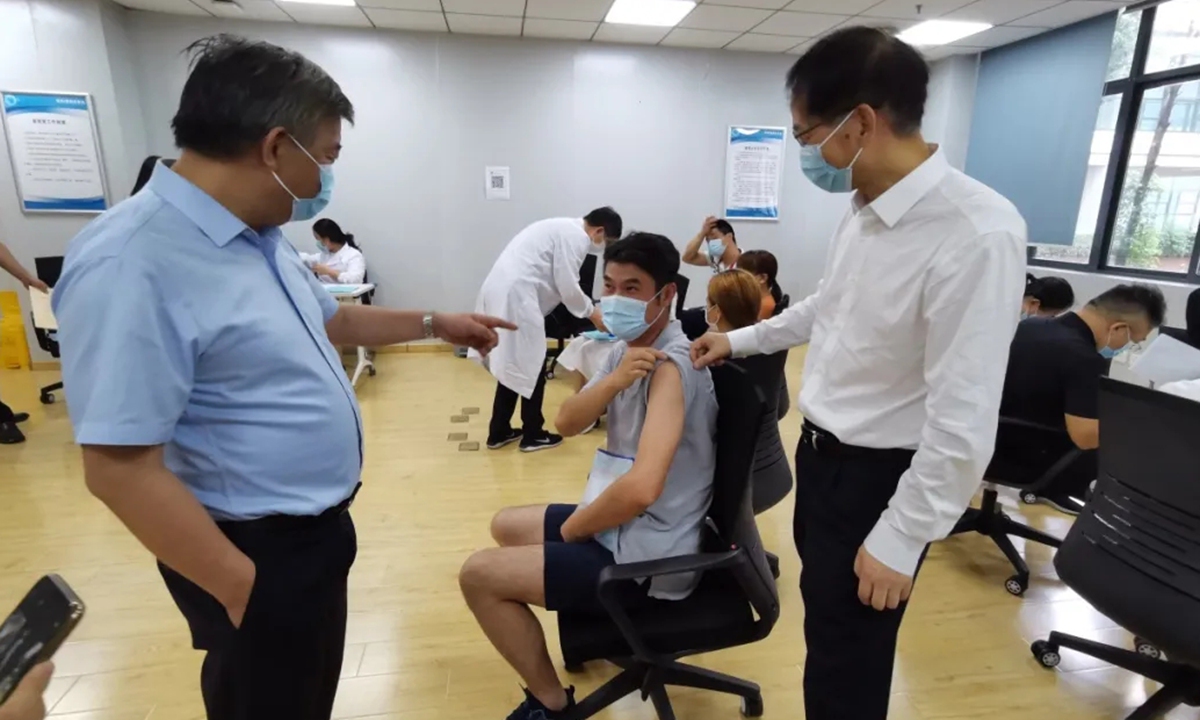
Volunteers receive China's first COVID-19 vaccine candidate from insect cells developed by the State Key Laboratory of Biological Therapy of Sichuan University-affiliated West China Hospital. Photo: Courtesy of the hospital
China's first recombinant protein COVID-19 vaccine made from insect cells was recently injected in volunteers, who said they felt good and have had no adverse reactions so far, the Global Times learned from the vaccine developer on Monday.
Developed by the State Key Laboratory of Biological Therapy of Sichuan University-affiliated West China Hospital, the vaccine uses insect cells to multiply in the culture medium, and introduces the gene of COVID-19 to insect cells, which means the cell can be used to produce high-quality recombinant vaccine proteins and purify them for refinement.
The vaccine was injected in several volunteers on Saturday morning, according to a hospital statement sent to the Global Times.
Academician Wei Yuquan, the director of the lab, said the vaccine was approved for clinical trials by the National Medical Products Administration on August 21 after being tested on monkeys and other animals. It was found to have a good protective effect against COVID-19 infection, with no obvious side effects.
It is easy to mass-produce the vaccine, Wei said.
So far, four types of coronavirus vaccines in China have started Phase III clinical trials, and some will be injected in volunteers in early September.
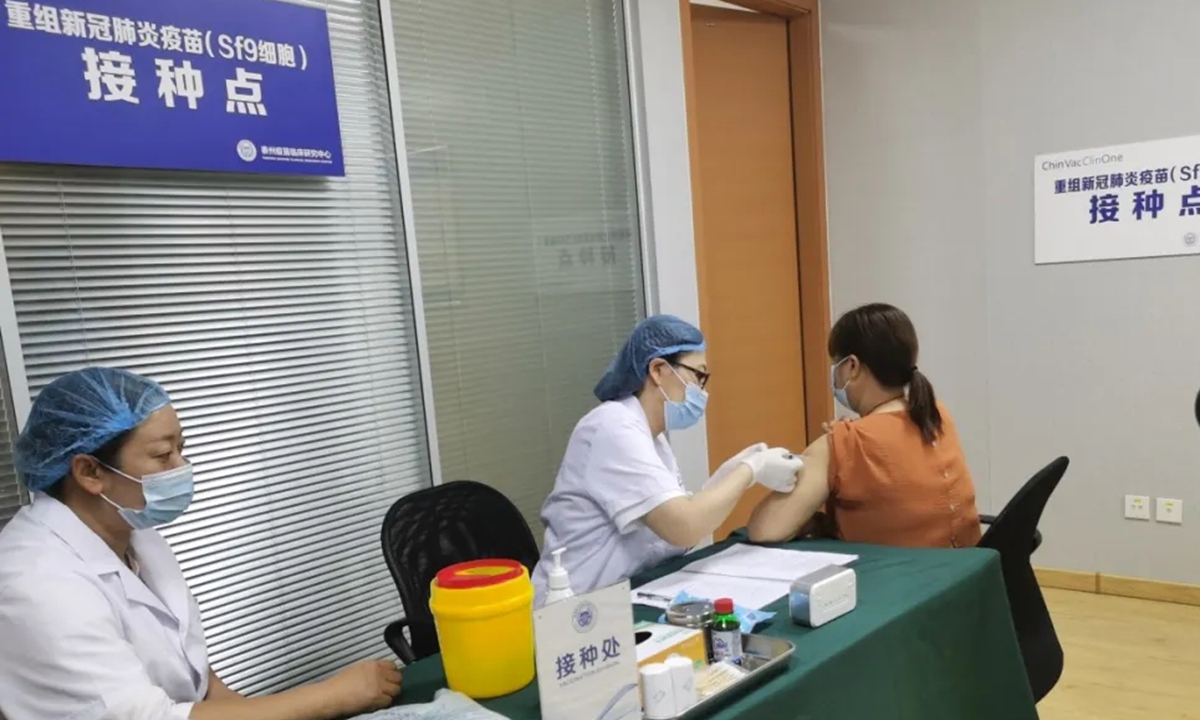
Volunteers receive China's first COVID-19 vaccine candidate from insect cells developed by the State Key Laboratory of Biological Therapy of Sichuan University-affiliated West China Hospital. Photo: Courtesy of the hospital
The Phase III clinical trials determine whether the vaccine would be approved. It will verify the vaccine's safety and effectiveness, which requires tens of thousands of samples.
Media reported that the phase 3 clinical trials are expected to achieve preliminary results as early as of November.
Results of Phase I and Phase II show several vaccines have been safe and effective.
A document published by the National Food and Drug Administration on August 15 said a novel coronavirus vaccine should be able to provide protection for at least six months.
There are five main technologies for the development of COVID-19 vaccine in China - inactivated vaccine, recombinant protein vaccine, adenovirus vector vaccine, nucleic acid vaccine based on mRNA and DNA, and attenuated influenza virus vector vaccine.
Developers around the world are racing against the clock with 170 vaccine candidates. Among those candidates, four from China, two from the US, and one from the UK have entered the last phase. Russia's vaccine, Sputnik V, has recently been under the spotlight after the country announced on August 11 that it had become the first in the world to approve a coronavirus vaccine for widespread use.

The State Key Laboratory of Biological Therapy of Sichuan University-affiliated West China Hospital starts research on COVID-19 vaccine made from insect cells. Photo: Courtesy of the hospital