The COVID-19 vaccine Beijing manufacturing plant of leading Chinese vaccine producer Sinovac Photo: Li Hao/GT
Editor's Note:Since China officially kicked off mass vaccination on December 15, 2020, more than 15 million people have been vaccinated as of Wednesday, according to a national health official. Chinese vaccines have also been purchased and used by more than 20 foreign countries. However, along with recognition and praise, doubts and smears over safety and efficacy of China's vaccines have never stopped. Are Chinese vaccines really not as good as foreign ones? Feng Duojia (Feng), president of the China Vaccine Industry Association, received an exclusive interview with the Global Times reporters Leng Shumei and Zhang Han (GT) on Wednesday.

Feng Duojia, president of the China Vaccine Industry Association
GT: You have said in previous interviews with media that Chinese people used to have a low willingness for inoculation. Do you think this will be a hurdle for China's mass vaccination in the future, or is it an opportunity to change Chinese people's attitude?
Feng: Take the flu vaccine for example, before the COVID-19 epidemic, Chinese people were not as willing to accept flu vaccines as people in Western countries.
But along with the popularization of vaccine knowledge in recent years, Chinese people's attitudes have also changed a lot, and the number of people willing to accept vaccines has gradually increased year by year. At times, supply even could not meet demand.
China's achievements in the epidemic control and prevention have demonstrated that Chinese people unite as one, work as one and have high consciousness about public health. Moreover, along with the popularization of the knowledge about vaccination, the public will certainly become more and more willing to accept vaccines. The smooth progress of the current mass vaccination in China is the best proof.
In fact, China has a good tradition of achieving herd immunity and overcome epidemics through vaccination. For example, through vaccination we have eliminated smallpox and polio, reduced the incidence and infection rate of hepatitis B, and brought measles under control.
Although some people are still hesitant to get COVID-19 vaccine shots, overall, the public have showed an all-time high vaccination willingness and stronger confidence in ending the COVID-19 epidemic.
A man receives COVID-19 vaccine in Dongcheng district, Beijing, on January 17. Photo: VCG
GT: Amid reports and concerns over adverse effects of Pfizer's mRNA vaccines, do you think it is necessary to adjust inoculation programs? Will the negative reports impact global vaccination progress?
Feng: Any vaccine, after being approved for market, will go through further evaluation which is dubbed as "Phase IV clinical trial" on masses of people who receive it to further study the safety and efficacy. The Pfizer vaccine is the first vaccine in the world that uses the mRNA route; mRNA route has been under development for more than 20 years. Its commercial use means it has finally entered the post-market evaluation phase.
As for the reports on adverse reactions and deaths, whether the adverse reactions exceed normal range and whether deaths are connected to the vaccine itself or its quality require professional analysis by scientists, medical experts and drug regulators.
Based on previous experiences, the difference between vaccines of various routes lies in the production process. The safety and efficacy of vaccines for the same pathogen do not have fundamental differences.
A military personnel receives COVID-19 vaccine from Chinese pharmaceutical firm Sinopharm in Belgrade, Serbia, on January 19. Photo: VCG
GT: Some Western media have doubted and smeared the vaccines of Chinese companies CNBG and Sinovac, claiming they have not reached as high efficacy as those of Pfizer and Moderna. What do you think about the difference in the efficacy of Chinese and foreign vaccines? How can Chinese companies increase international market's trust in Chinese vaccines? What are the challenges Chinese companies face?
Feng: Efficacy of various vaccines is not necessarily comparable due to the difference in clinical trial design, sites, population and statistical method. These vaccines' safety and efficacy are guaranteed as long as the clinical trial results are in accordance with the World Health Organization (WHO)'s guidance and basic standards as well as with national regulations, and have been strictly reviewed and evaluated. There is no fundamental difference in the efficacy.
Whether Chinese vaccines can win public trust in the international market depends on the following factors: first, public trust in the Chinese government and its international commitments; second, performance of Chinese vaccines in China's epidemic prevention; third, international quality approval; forth, performance of Chinese vaccines in foreign countries in preventing epidemic; and fifth, the supply rate and service quality.
Chinese vaccines have gained high public trust in the aspects mentioned above.
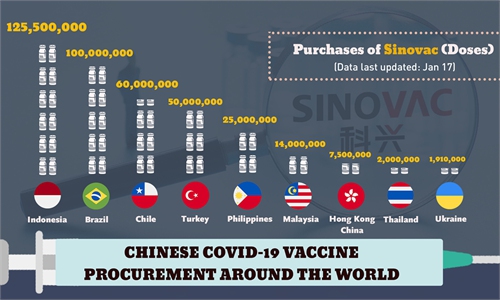
Chinese President Xi Jinping promised to make "China's contribution to ensuring vaccine accessibility and affordability in developing countries" at the World Health Assembly [in May 2020]; China's epidemic control results have won praise of the WHO; four Chinese vaccine producers have obtained the WHO pre-certification; Chinese COVID-19 vaccines have been purchased by more than 20 countries and are being rolled out in various countries; and China has joined COVAX jointly initiated by the WHO and the Global Alliance for Vaccines and Immunization. The fact that many senior officials and politicians in various countries have been the first in their countries to get shots of Chinese vaccines is a demonstration of public trust in China's vaccines.
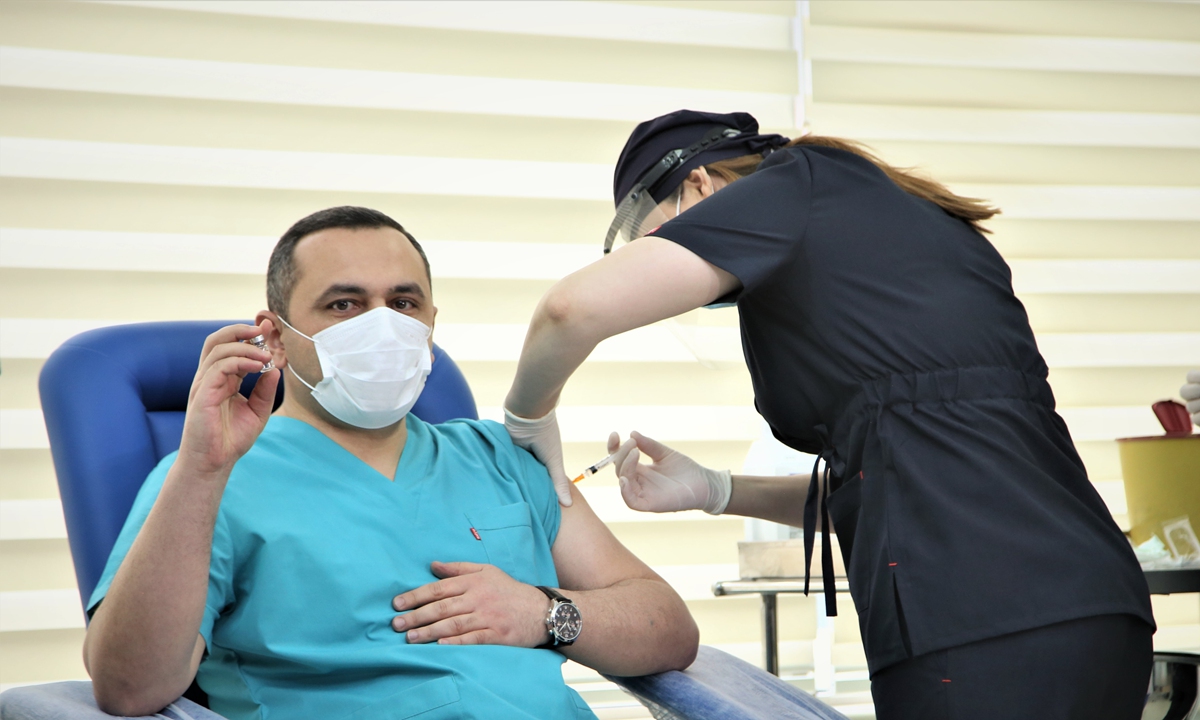
A man receives CoronaVac on the first day after Azerbaijan started COVID-19 vaccination, in Baku, Azerbaijan, on January 18. Photo: VCG
GT: Do you think China needs to achieve herd immunity despite current achievements in the epidemic prevention and control? Considering China's vaccine production capacity, when do you think can we build up herd immunity? Will there be an immunity gap between China and other countries as some experts have warned?
Feng: Although we have made huge achievements in the epidemic prevention and control, we still have to build up herd immunity through vaccination as the pandemic situation is still serious globally. To build up herd immunity, we have to follow the rules of preventive medicine and reach a certain rate of population coverage.
China's vaccination work is in accordance with global pace and the progress is in line with the schedule, so there will not be a so-called immunity gap.
As to when we could reach herd immunity, it depends on the rate and quantity of supply and vaccination schedule.
Related authorities will also adjust vaccination schedule and strategy according to the trend of the epidemic.
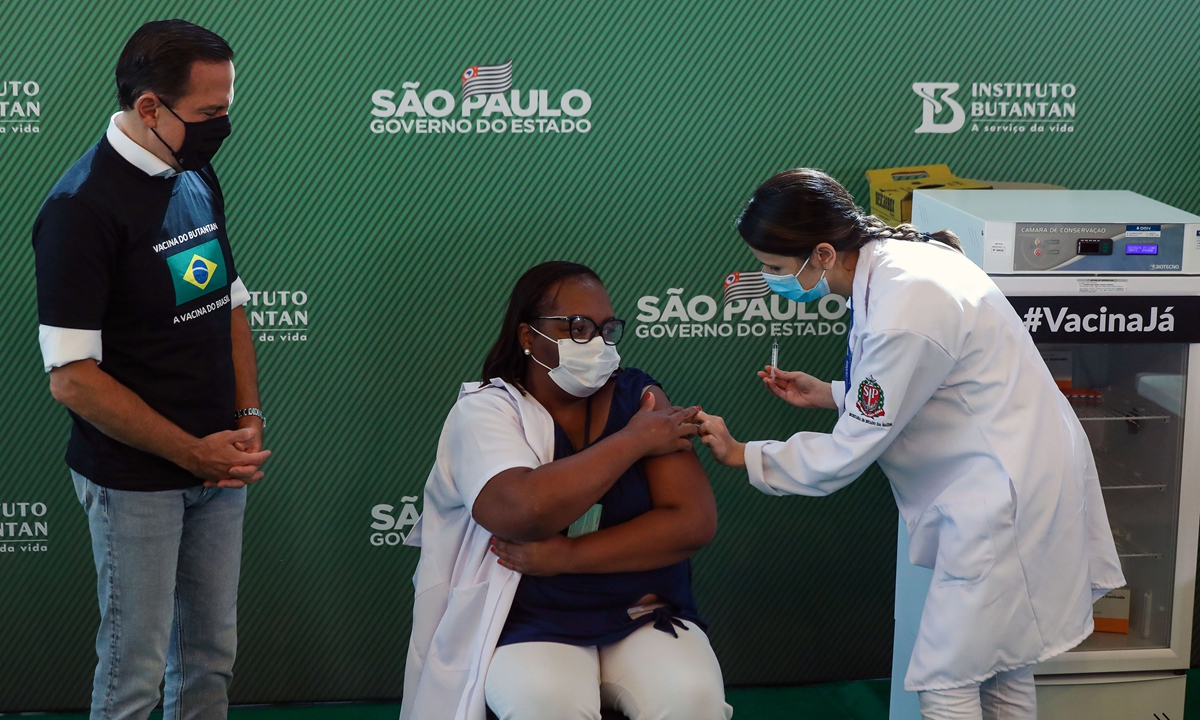
A nurse, 54, receives the first CoronaVac shot in Brazil on January 17. Photo: VCG
GT: The mRNA route is based on a new technology to develop vaccines. How is China's performance on this route?Feng: China has mRNA as one of its five technology routes to develop COVID-19 vaccines. Two companies' vaccines have entered clinical trials, suggesting they have mastered such technology, and they have begun construction of facilities for mass production.
GT: What kind of materials or equipment does China lack to achieve self-reliance in vaccine production? What kind of potential does China have?
Feng: Overseas producers have occupied most of the market for cell culture medium with their advantages in development, quality control and brand awareness. But the booming bio industry in China has boosted domestic brands.
For pre-filled syringe and vial materials (glass tubes), China had to depend on a lot of imports. But the short supply and logistical bottlenecks due to the pandemic have pushed Chinese firms to achieve self-reliance in the entire supply chain, and they have made good progress.
China used to rely heavily on some equipment for vaccine production, such as culture tanks, purification facilities and digital management systems. But now domestic producers can not only supply these to the domestic market, but also export.
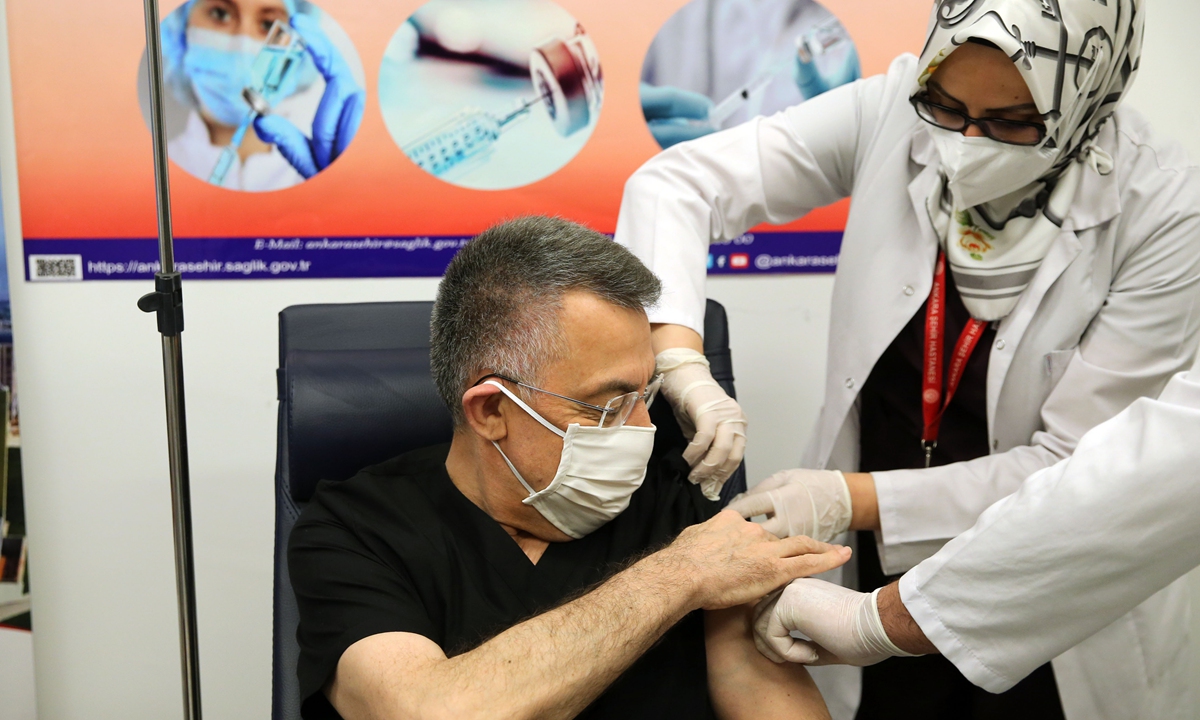
Turkish Vice President Fuat Oktay receives China's CoronaVac in Ankara City Hospital, Ankara, Turkey, on January 16. Photo: VCG
According to China's immunity plan, we need to inoculate 1 billion people, which means 2 billion doses. And the demand may increase to 4 billion if we add supplies for other developing countries. Eighteen vaccine producers are accelerating expansion of manufacturing capabilities, and China's total annual capacity is expected to reach 2 billion doses by the end of 2021.
With more developers getting regulator's approval for their vaccines and building the production capacity, the nation's total capacity will eventually reach 4 billion per year, covering 40 percent of world demand, based on calculations of world population and average vaccination rates.