Sinovac photo: Li Hao/GT
Promising interim data released by China's COVID-19 developer Sinovac regarding its vaccines on elderly people will push forward China's efforts to streamline its vaccine rollout for seniors and consolidate the country's plan to establish herd immunity. Yet scientists urged caution and incremental administration of inoculations on this vulnerable group, after having witnessed adverse reactions and deaths in Western countries suspected to be caused by vaccinations.
Sinovac has released Phase-I and Phase-II clinical trial data on healthy adults aged 60 years old and above which shows the vaccine will be safe and stable in older adults, according to a document Sinovac sent to the Global Times on Thursday.
The Phase-I and Phase-II clinical trials by Sinovac have included 422 adults aged 60 years and older in Renqiu city, North China's Hebei Province between May and June 2020.
Vaccine efficacy is usually reduced in older adults due to their failing immune systems, but findings showed Sinovac's vaccine is well tolerated by the body and immunogenic in healthy adults aged 60 years and older, with neutralizing antibody responses to live coronavirus not reduced in this key group.
All adverse reactions were of mild or moderate severity. The most frequently reported reactions were injection site pain, which occurred among 39 participants, and fever, which occurred among 14 people. Most of the adverse reactions occurred within seven days of vaccination and participants recovered within 48 hours.
Sinovac's findings were released in the Lancet medical journal on Wednesday. It was the first report of an inactivated SARS-CoV-2 vaccine tested in adults aged 60 years old and above.
This was hailed by Chinese vaccine experts as a good sign that heralds approval and expansion of the use of the COVID-19 vaccine in elderly groups.
Feng Duojia, president of the China Vaccine Industry Association, told the Global Times on Thursday that Phase-III data and approval from drug authorities are needed for elderly people to be vaccinated.
The Phase-II data for senior adults cannot represent the Phase-III efficacy data, because the Phase-II trial primarily monitored antibody levels, whereas the Phase-III data evaluated the infection risk reduction levels through assessing the number of COVID-19 cases in the double-blind, placebo-controlled trials, a representative of Sinovac told the Global Times on Thursday.
Feng noted that Sinovac and Sinopharm, another Chinese COVID-19 vaccine producer which has already been granted conditional market approval, are both likely to announce their Phase-III data on elderly and juvenile groups around the Chinese New Year holidays, which begins on February 12.
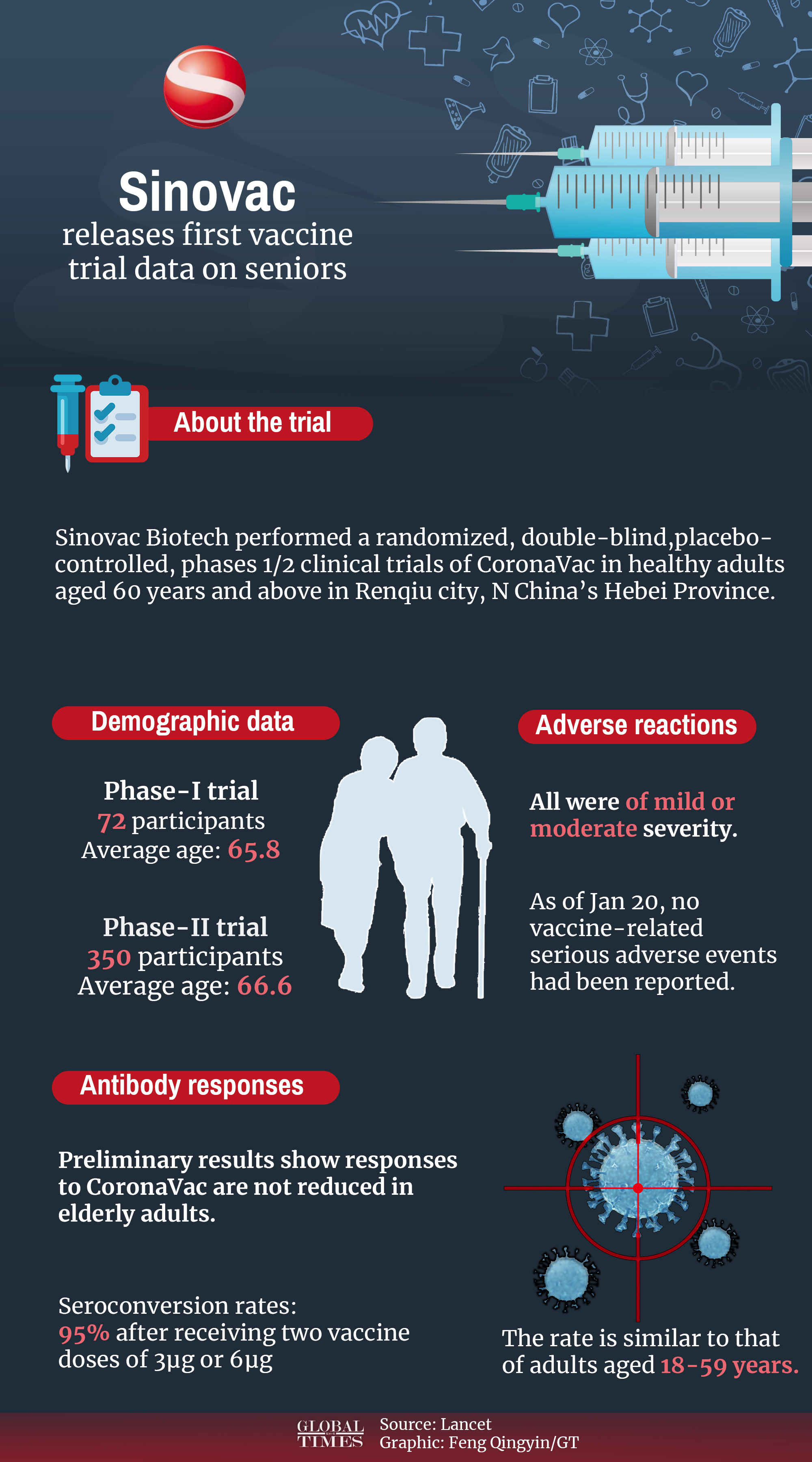
Sinovac vaccines induced neutralizing antibody responses to live novel coronavirus in adults aged 60 and above, according to the results from the Phase-I and Phase-II clinical trials. All adverse reactions observed among elderly groups were of mild or moderate severity. Graphic: Feng Qingyin/GT
Cautiously stepping forward
Jiang Chunlai, a professor from Jilin University's School of Life Sciences, told the Global Times on Thursday that as a normal procedure to push forward vaccines, elderly groups should have been categorized into the first tier of vaccinations.
Yet, he noted that caution remains for China to push forward the vaccination of the elderly, as the seniors actually have significantly lower levels of early side effects. "Also, clinical trials on the elderly should be put under more scrutiny," said Jiang.
Scientists also cited examples of recent deaths among elderly people in Norway in mid-January after having inoculated vaccines against COVID-19.
Later, in order to address fears regarding having the vaccine administered, The Norwegian Medicines Agency said there was no evidence of a direct link between the recent string of deaths and the vaccinations.
Yet within one month, media reported on Monday that all 78 residents at a nursing home in Madrid, Spain had tested positive for COVID-19 after being given their first dose of the Pfizer-BioNTech vaccine on January 13, and at least seven people have died.
Yang Zhanqiu, a deputy director of the pathogen biology department at Wuhan University, believes that unlike the mRNA vaccines used in European countries, China's inactivated vaccine is the safest for elderly people, given its mature technology in its applications on vaccines and little recorded adverse effects.
There is little chance of having extreme occurrences such as death with the use of inactivated coronavirus vaccines, according to Yang.
Unlike other studies of the vaccine, Brazil's Sinovac trial included elderly volunteers.
Dimas Covas, director of Brazilian biomedical center Butantan, Sinovac's research and production partner, said in January that the Sinovac vaccine had entirely prevented severe COVID-19 cases among vaccinated groups, including in the elderly group. None of those who received the vaccine became ill enough to require hospitalization, he said.
Another Chinese COVID-19 vaccine producer CanSino Biologics, which used Ad5-nCOV vaccine, announced it has met its pre-specified primary safety and efficacy criteria at interim analysis. The Ad5-nCoV's Phase-II and Phase-III trials have involved elderly participants, with no serious adverse reactions reported, the Global Times learned on Monday from a source close to CanSino.
"The mRNA approach could be the worst recommended vaccine for the elderly group," Yang said.
He suggested a step-by-step vaccination process for mass inoculation of the seniors. "First, covering those elderly people working in high-risk positions, such as medical workers, and then to senior residents in places with previous outbreaks, such as North China's Hebei and Northeast China's Jilin provinces. The last step will be to administer the vaccine to other elderly people."
Global vaccine shepherd
Several Chinese vaccine producers have made impressive breakthroughs this week, which was hailed by experts as not only consolidating China's position as a global vaccine R&D shepherd, but also offering a glimmer of hope in the global battle against COVID-19.
On Wednesday, Sinovac's application for the conditional market launch of its inactivated COVID-19 vaccine was accepted by China's drug regulator, which is seen as a major step forward in making Chinese vaccines available for public use.
Chinese pharmaceutical company Fosun Pharma, a partner of German Biotech company BioNTech, is on track to seek approval from the Chinese regulator for the COVID-19 mRNA vaccine to be used in China, Hui Aimin, president of Global R&D and Chief Medical Officer of Fosun Pharma, told the Global Times in an exclusive interview on Wednesday, noting that he is "very optimistic" about the mRNA vaccine development in China.
China has administered more than 31 million doses of COVID-19 vaccines on key groups as of Wednesday, Mi Feng, spokesperson from China's National Health Commission, said in a Thursday conference.
Meanwhile, China is generously offering vaccines to countries in urgent need. As per the request of the WHO, China will provide 10 million doses of COVID-19 vaccines for emergency use in developing countries, Wang Wenbin, spokesperson of China's Ministry of Foreign Affairs, said on Wednesday.
China always views its vaccines as a kind of global public goods, and is willing to cooperate with other countries on R&D of COVID-19 vaccines, said Feng, noting the fact that increasingly more countries have casted their vote of confidence toward Chinese vaccines, helping consolidate China's status as a "guaranteed fair distributor of global vaccines."