Sinopharm's COVID-19 vaccine receives 1st EU GMP certificate from Hungary, 'to be more competitive in intl market'
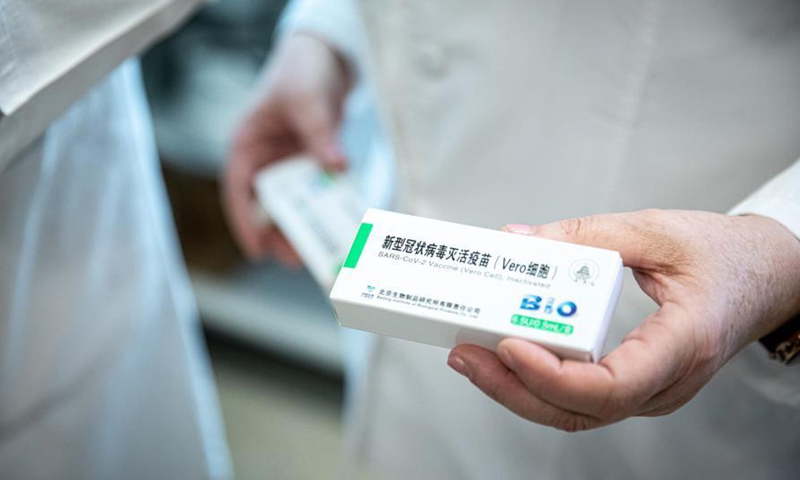
A box of China's Sinopharm COVID-19 vaccine is seen in Budapest, Hungary, Feb. 28, 2021. Photo:Xinhua
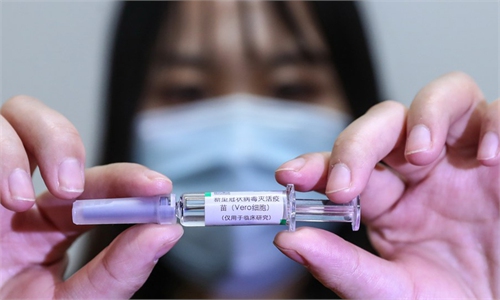
China National Pharmaceutical Group Co., Ltd. (Sinopharm) announced on Saturday that the COVID-19 vaccine produced by its Beijing institute has been issued a Good Manufacturing Practice (GMP) certificate by Hungarian authorities, marking the first for a Chinese COVID-19 vaccine receiving such a certificate from an EU country and a step forward for Chinese vaccines to become a global public goods.
Vaccine experts pointed out that the issuance of this certificate will greatly enhance the competitiveness of China-produced vaccines in Europe and the confidence of small European countries in Chinese vaccines given the uneven distribution of vaccines in Europe.
Sinopharm announced on Saturday that it acquired the GMP certificate issued by the Hungarian National Institute of Pharmacy and Nutrition on Thursday.
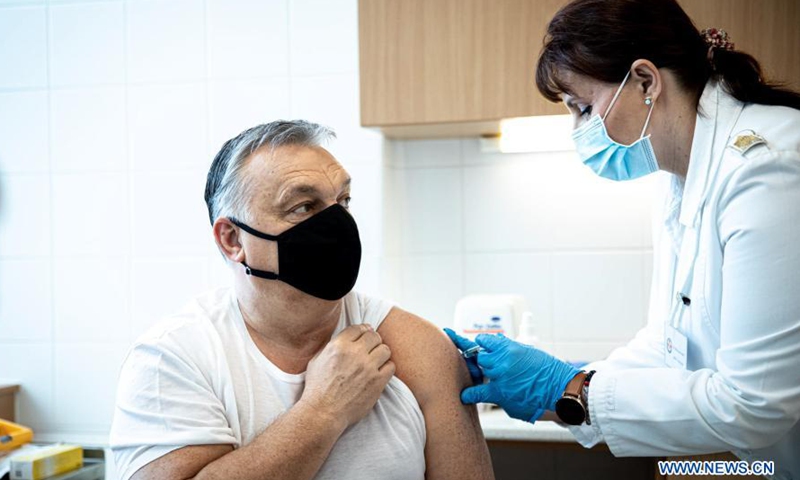
This photo from Facebook page of Hungarian Prime Minister Viktor Orban shows him receiving a dose of China's Sinopharm vaccine against COVID-19 in Budapest, Hungary, on Feb. 28, 2021. Photo:Xinhua
Sinopharm COVID-19 vaccine is also the first Chinese vaccine authorized for emergency use by the EU, according to a company statement.
The GMP is a system for ensuring that products are consistently produced and controlled according to quality standards. It is designed to minimize the risks involved in any pharmaceutical production that cannot be eliminated through testing the final product, according to the International Society for Pharmaceutical Engineering.
The Hungarian National Institute of Pharmacy and Nutrition has reviewed the production of the Sinopharm vaccine and obtained the full documentation on production processes in January. On March 3, Sinopharm submitted the relevant follow-up report to the relevant Hungarian drug regulator. On Thursday, Hungary officially issued a GMP certificate to Sinopharm's COVID-19 vaccine, in accordance with EU regulatory standards and rules.
Jiang Chunlai, a professor at Jilin University's School of Life Sciences, told the Global Times on Saturday that the grant of the certificate will enhance Chinese vaccines' competitiveness in the international market.
In addition to competitiveness, the certificate also facilitates increased confidence in Chinese vaccines in small European countries, Feng Duojia, president of the China Vaccine Industry Association, told the Global Times on Saturday.
The certification of Chinese vaccines in an EU country also reflects the increased confidence of some EU countries in Chinese vaccines and the demand for Chinese vaccines under the current situation of insufficient and uneven distribution of vaccines within the EU, Feng said.

Boxes of the Sinopharm COVID-19 vaccine are distributed to family doctors for vaccination in Kecskemet, Hungary on Feb. 24, 2021. Photo: Xinhua
"This decision made by Hungary can provide a reference for the future approval of Chinese vaccines in other EU countries, which will also lead to more recognition of Chinese vaccines in the EU," Feng noted.
100 million doses of COVID-19 vaccines from Sinopharm have been supplied worldwide. Hungary started mass vaccinations with Sinopharm shots in late February, the first EU country to use Chinese COVID-19 vaccine. For several weeks now, no serious adverse reactions have been reported in Hungary, which has earned the Sinopharm vaccine trust in the European country.
China is accelerating its nationwide inoculation drive with some places vowing to vaccinate about 40 percent of their local population by June.
As of Friday, a total of 133.8 million doses have been administered on residents in the Chinese mainland. According to Our World in Data, a data tracking website, the US has administered a total of 157.61 million doses as of Friday.
Tao Lina, a Shanghai-based vaccine expert, said that it is certain that China can surpass the US in the first half of next week regarding the number of administered doses.