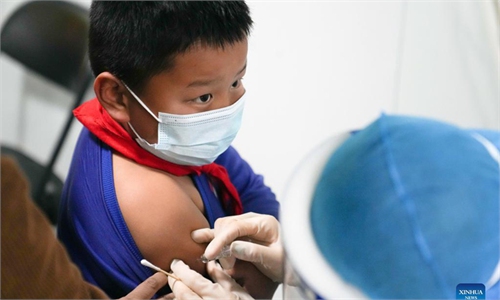
Volunteer of world's first dose of inactivated vaccine against Omicron shows no physical discomfort
Photo: VCG
The first dose of COVID-19 inactivated vaccine specifically developed against the Omicron variant was administered on Sunday during a clinical trial in Hangzhou, East China's Zhejiang Province, which brings much hope to the country where many are suffering from the highly transmissible virus.
After undergoing relevant testing, informed consent, basic physical examination and sample collection, volunteers received the inactivated vaccine at Shulan Hospital in Hangzhou.
One of the volunteers told the Global Times on condition of anonymity that he chose to be one of the first volunteers in the clinical trial of this vaccine because he wanted to "make a small contribution" to the development of vaccines.
A staff member at the vaccination site told the Global Times that the volunteer did not show any physical discomfort after receiving the shot.
Due to the strong infection rate and fast transmission, the Omicron variant is very concealed. The average time of infection is shorter and those catching the virus are mostly asymptomatic. These characteristics make epidemic prevention and control more difficult. Many outbreaks across China were related to the Omicron strain.
In this context, China's vaccine manufacturer Sinopharm developed a specific vaccine against Omicron which was approved for sequential immunological clinical studies in Hong Kong on April 13, becoming the first inactivated vaccine against Omicron for clinical use in the world.
On April 26, the vaccine was approved by the State Drug Administration and entered the stage of clinical research in the mainland.
The volunteer told the Global Times that, as worker in the health industry, he has been paying attention to the development of vaccines. Having witnessed the fast spread of the Omicron variant, he decided to participate in the clinical research.
The volunteer revealed that participants have to be over 18 years old and without any vaccine against COVID-19.
The Omicron-specific vaccine candidate developed by Sinopharm can trigger high levels of neutralizing antibodies against the variant while enhancing protection against other main strains by two to three times, Zhang Yuntao, one of chief scientists from the China National Biotec Group (CNBG), revealed at a press conference on April 28.
"The clinical trials in the mainland and in Hong Kong could take an estimated three to four months," Zhang mentioned.
In a previous announcement of the approval of the clinical trials, the CNBG said that the tests will be conducted on subjects aged 18 and above with two or three shots of vaccines to evaluate the safety and immunogenicity of the Omicron-specific candidate.
Cross neutralization tests in vitro showed that the CNBG's Omicron-specific candidate can trigger very high levels of neutralizing antibodies against the Omicron variant, Zhang said, noting that pre-clinical trials indicated that the candidate shot is effective against the Omicron variant.
The candidate shots could also enhance neutralizing antibodies against the original strain of the virus and the Delta and Beta variants by two to three times, Zhang noted.
Yang Huichuan, chief scientist from the CNBG, said during the press conference that the company had built six manufacturing plants and three laboratories with protection level III. If the Omicron candidate vaccine succeeds at last, the CNBG will devote most manufacturing facilities to its production.