Sinopharm's F61 nasal spray receives clinical trial approval for people at high risk of exposure to COVID-19
Sinopharm Photo: VCG
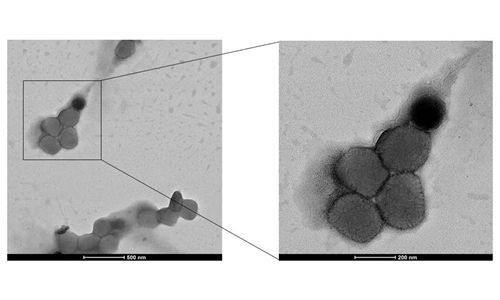
The recombinant human monoclonal antibody nasal spray against SARS-CoV-2 (F61 Nasal Spray) of Sinopharm's Wuhan Institute of Biological Products has received approval for clinical trials from the National Medical Products Administration (NMPA) for people at high risk of exposure to the novel coronavirus, the company announced on Monday.
The approval is of great significance as it will be an important weapon in the fight against the rapidly spreading Omicron variant.
According to the company, the F61 monoclonal antibody showed broad-spectrum neutralizing activity against SARS-CoV-2 prototype strains and major variants against COVID-19. In particular, it maintained high activity against Omicron BA.1, BA.1, BA.2, BA.3, BA.4/5 and BF.7, which were the main strains of the current epidemic.
Research into the F61 nasal spray was carried out at the same time as the F61 recombinant human broad-spectrum monoclonal antibody injection of the Wuhan Institute received clinical trial approval from the NMPA on July 22.
The F61 nasal spray uses the same production process and preparation prescription as the F61 injection, and has a broad spectrum and high level of activity against all variants of the novel coronavirus.
The F61 nasal spray is easy to use and can directly act on the nasopharyngeal upper respiratory tract, forming a protective membrane on the nasal mucosa, the company said. When the novel coronavirus invades, this protective membrane can prevent it from binding with host cells, specifically blocking the invasion of the novel coronavirus.
Previous real-world studies have shown that it is safe and effective in people exposed to the novel coronavirus in many places across the country, and can effectively reduce the positive rate of people who have come in close contact.
At present, the COVID-19 epidemic situation at home and abroad is still quite severe, and there is an urgent need for effective epidemic prevention and control products.
According to the company, the F61 nasal spray for preventive use is a product with independent intellectual property rights that can be used to protect people at high risk of exposure to the novel coronavirus, and to protect people's lives and health.
Global Times