Sinopharm's COVID-19 vaccine. Photo: VCG
China's leading pharmaceutical giant China National Pharmaceutical Group (Sinopharm) revealed that it has developed four potential Omicron-specific candidate vaccines, as experts call for quicker approval of such candidates, highlighting risk of surging cases during holiday.
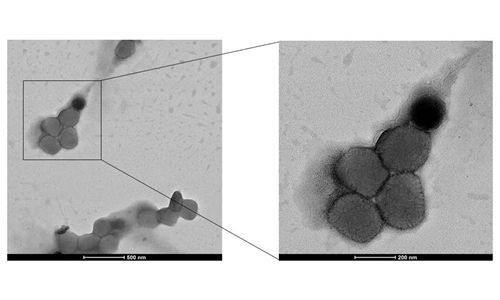
Liu Jingzhen, Sinopharm chairman, said in an interview with China Central Television (CCTV) on Wednesday that the group had developed four Omicron-specific candidate vaccines including inactivated, recombinant protein, and mRNA varieties, as well as a very effective preventative inhaled monoclonal antibody product.
The inhaled monoclonal antibody is effective in preventing the virus for two or three days, according to Liu.
Liu said that he had already taken two shots of the group's Omicron-specific vaccines. But these candidates still have to undergo an official review and approval procedure before being provided to the public, Liu noted.
Liu's announcement came as Gao Fu, former head of the Chinese Center for Disease Control and Prevention (CDC), suggested Wednesday that vaccines against mutant strains of COVID-19 should be approved for use as soon as possible. Gao made the remarks during an interview with China Newsweek.
Considering the risk of local infections of Omicron XBB and its subvariants, and the Spring Festival travel rush involving over 2 billion trips, the largest in scale over the past three years, the rise of infection may appear in some rural areas due to the return of villagers, said Gao.
Although breakthrough infections are common, vaccinations can still provide protection. Whether for the elderly or young people, booster shots are still necessary after infection, Gao noted.
China has witnessed an explosion of COVID-19 cases on a scale that never happened in this country in the past three years, after the authorities downgraded epidemic management in December. China's National Health Commission (NHC) announced on January 14 a total of 59,938 COVID-19-related deaths between December 8, 2022 and January 12 this year.
Hyping the rising infections and deaths, some foreign media spread rumors and misinformation about the efficacy and safety of China-developed and -manufactured vaccines, mainly the inactivated vaccines of Sinopharm and Sinovac Biotech (SINOVAC).
In response to these rumors and misconceptions, SINOVAC clarified in materials it sent to the Global Times that research and real world data had proved the efficacy and safety of its vaccine, CoronaVac.
Research in Hong Kong showed that CoronaVac offered similar protection against severe disease and death as mRNA vaccines, the materials noted, citing a preprint released in November 2022 in medRvix.
Three and four doses of BNT162b2 or CoronaVac were effective against Omicron infection seven days after vaccination, the paper said. The findings suggest that booster doses can temporarily enhance population immunity ahead of anticipated waves of infections.
Liu also noted in the interview with CCTV that Sinopharm's inactivated COVID-19 vaccines had been approved by more than 119 nations, regions and international organizations since December 2020.
More than 3.5 billion doses of Sinopharm's vaccines had been produced and provided by the group to nearly 200 nations and regions so far, which observers said proved the vaccines' efficacy and safety, and squashed foreign media's rumors.